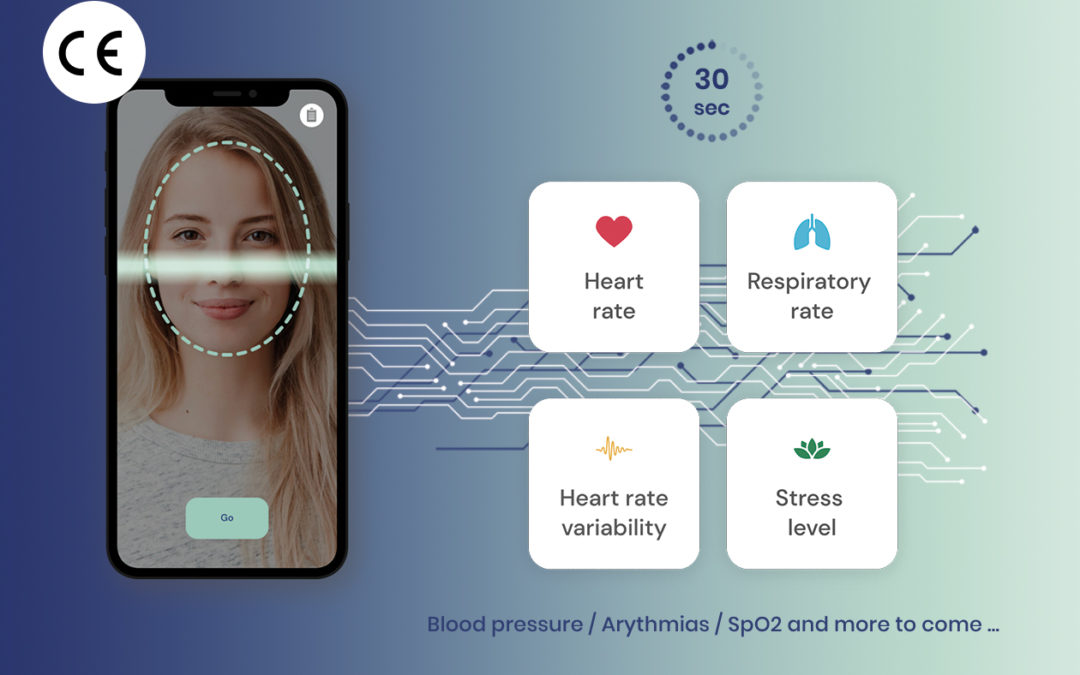
Caducy obtains CE certification as a medical device Class IIa
We are pleased to announce that our solution Caducy is now certified as a medical device Class IIa in conformity with the MDR (EU) 2017/745. It indicates that Caducy has met the safety, health and environmental requirements set by the European Union.
This world premiere is the result of a lot of hard work and determination from all the people referring to this project.
More information on this certification here.